Radioligand Therapy (Prostate) — Overview
High-level, prostate-focused overview of radioligand options and evidence notes. Pluvicto® is the regulatory anchor; other agents are included for landscape context.
Browse
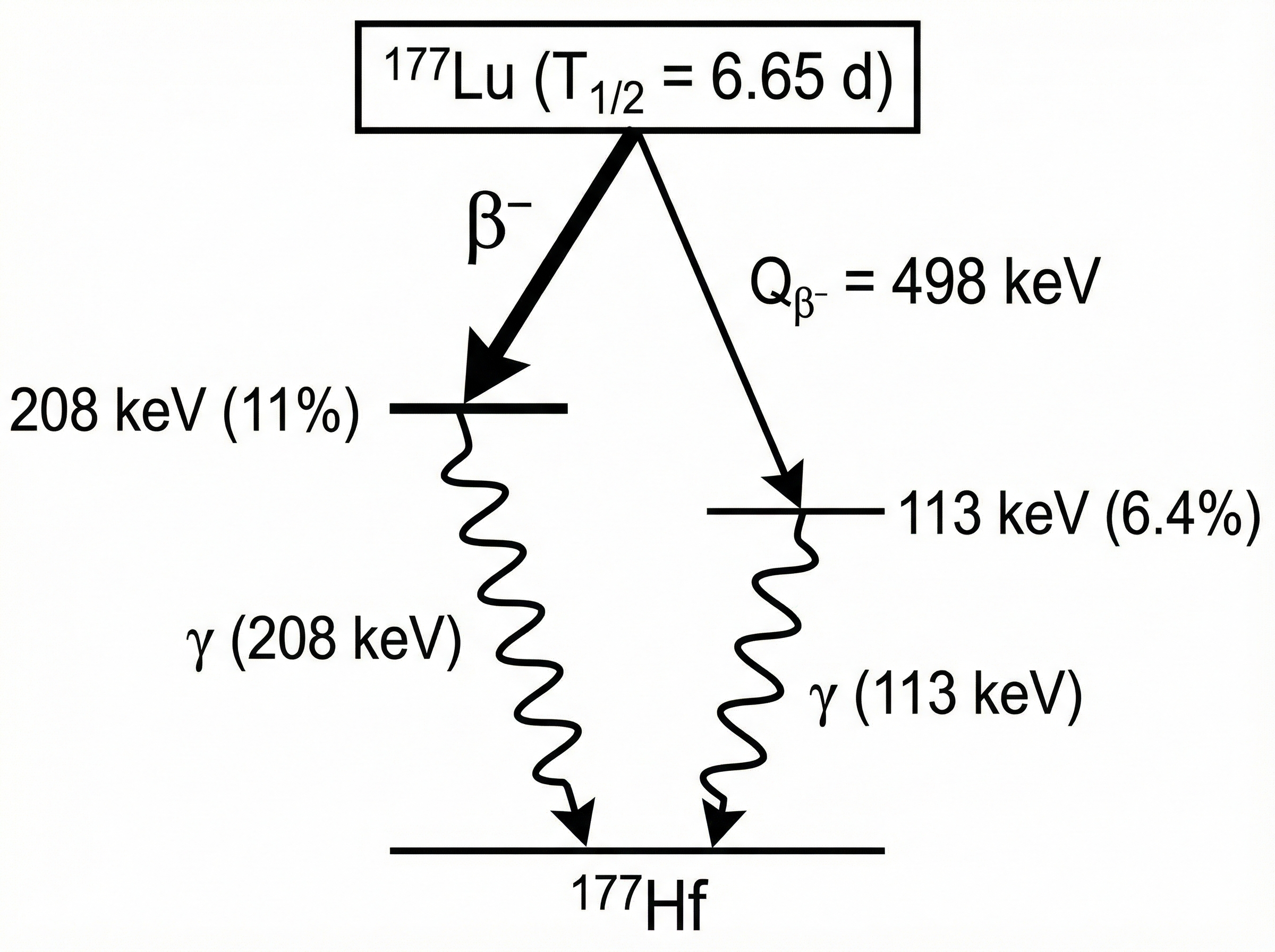
[177Lu]Lu-PSMA-617 (Pluvicto®)
Emitter: β
Status: FDA/EMA approved
Where it fits: mCRPC; post ARPI + taxane (per label/trials)
Toxicity signal: xerostomia; cytopenias
Evidence notes
- VISION reported OS/PFS benefit vs SOC in PSMA-positive mCRPC (see primary publication).
- Monitor per institutional protocol.
[177Lu]Lu-PSMA-I&T
Emitter: β
Status: Clinical use / trials (region-dependent)
Where it fits: PSMA-targeted alternative ligand
Toxicity signal: xerostomia; cytopenias
Evidence notes
- Comparative statements depend on population, imaging criteria, and trial design.
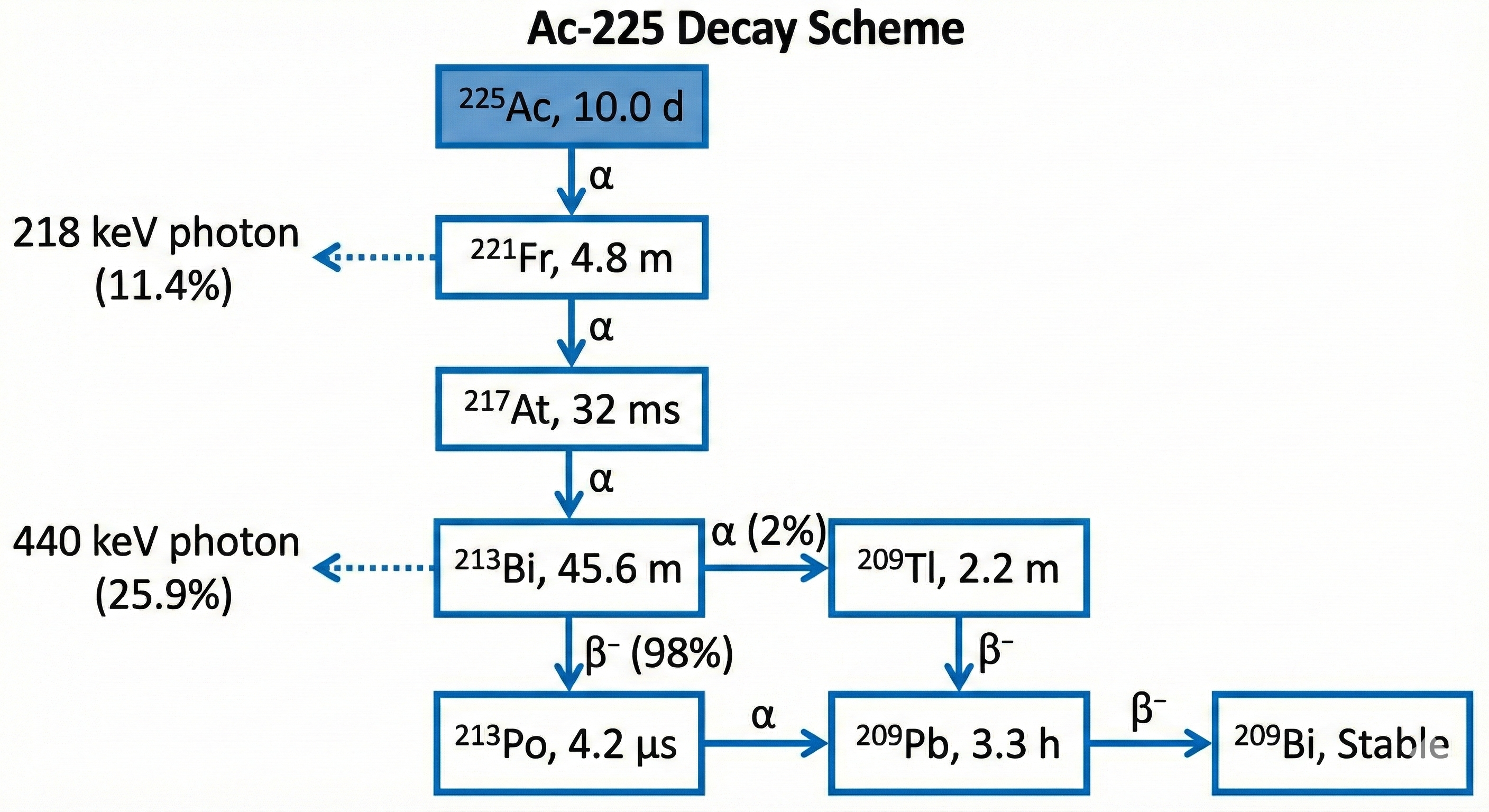
[225Ac]Ac-PSMA-617
Emitter: α
Status: Investigational / compassionate use
Where it fits: post-Lu progression (investigational); high-burden scenarios (series-level)
Toxicity signal: xerostomia (often more severe); cytopenias
Evidence notes
- Reported PSA responses in small series; interpret cautiously (selection bias; heterogeneous dosing).
- Xerostomia signal is prominent across published reports.
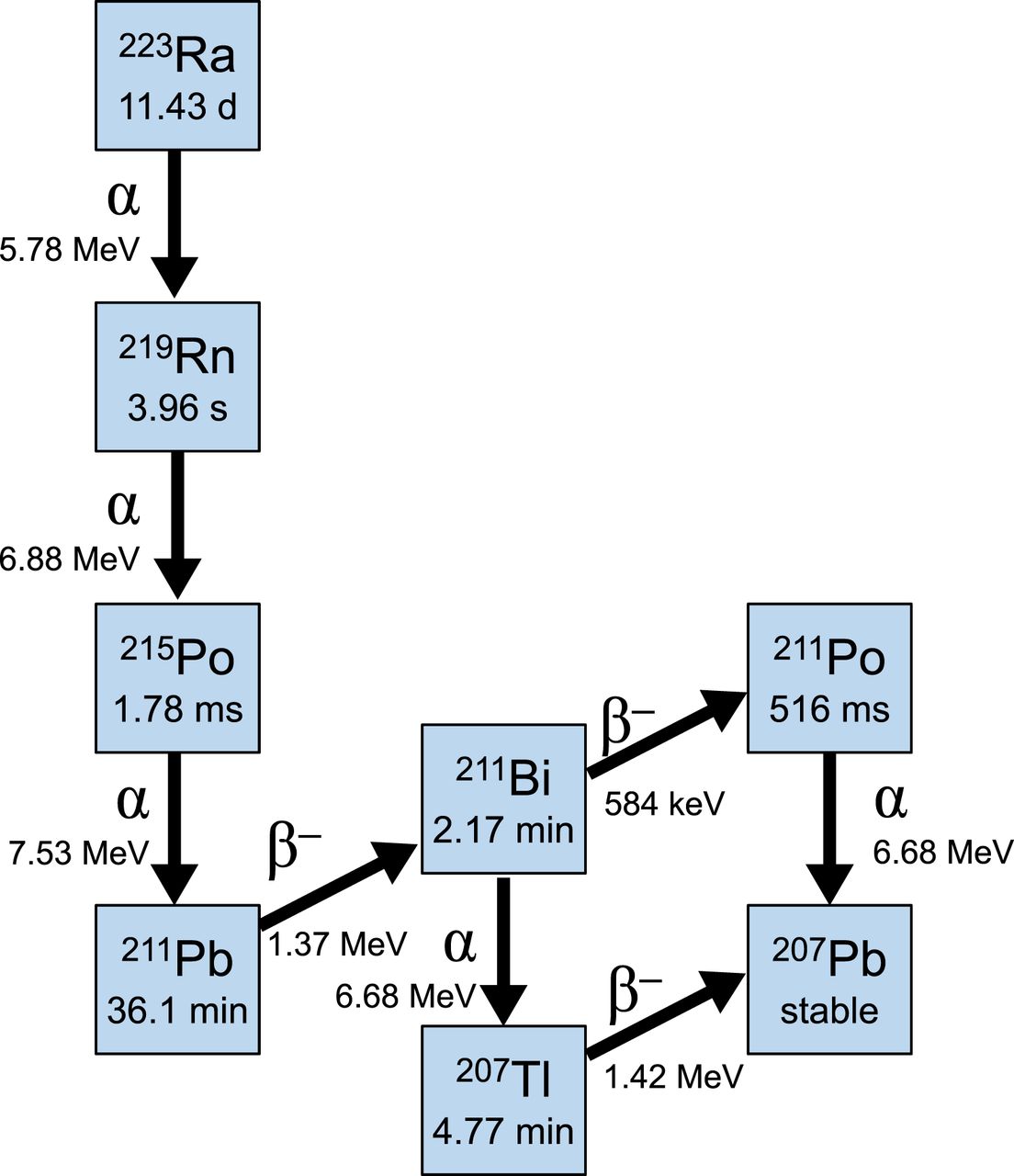
Ra-223 (Xofigo®)
Emitter: α
Status: FDA approved
Where it fits: symptomatic bone mets; no known visceral disease (per indication)
Toxicity signal: myelosuppression / thrombocytopenia
Evidence notes
- ALSYMPCA reported OS benefit and SSE delay in appropriate patients (see primary publication).
[131I]I-PSMA (MIP-1095 / I-PSMA-1095)
Emitter: β/γ
Status: Trials
Where it fits: PSMA-targeted (trial setting)
Toxicity signal: hematologic toxicity signal
J591 (PSMA monoclonal antibody, radio-labeled)
Status: Trials
Note: Antibody PK may shift marrow exposure; interpret within trial context.
Toxicity signal: marrow toxicity signal
Emerging PSMA ligands (albumin-binding, novel scaffolds)
Status: Early clinical / preclinical
Goal: PK engineering to alter tumor uptake / residence time
Toxicity: construct-dependent
| Agent | Emitter | Status | Where it fits (tags) | Key toxicity signal |
|---|---|---|---|---|
| [177Lu]Lu-PSMA-617 (Pluvicto®) | β | FDA/EMA approved | mCRPC; post ARPI + taxane | Xerostomia; cytopenias |
| [177Lu]Lu-PSMA-I&T | β | Clinical use / trials | PSMA-targeted alternative ligand | Xerostomia; cytopenias |
| [225Ac]Ac-PSMA-617 | α | Investigational | post-Lu progression (series-level) | Xerostomia; cytopenias |
| Ra-223 (Xofigo®) | α | FDA approved | Bone mets; no known visceral disease | Thrombocytopenia |
| [131I]I-PSMA (MIP-1095) | β/γ | Trials | PSMA-targeted (trial setting) | Hematologic toxicity |
| J591 (radio-labeled) | varies | Trials | Antibody-based targeting | Marrow toxicity |
This comparison is intentionally high-level and non-prescriptive.
Before considering PSMA-targeted RLT
- Confirm PSMA expression with appropriate imaging (document criteria used).
- Baseline marrow reserve (CBC) and prior marrow-toxic therapies.
- Renal function context (document baseline and monitoring plan).
- Discuss xerostomia risk (especially for alpha-emitter pathways).
- Clarify disease context and prior lines of therapy (for trial/label alignment).
Before considering Ra-223
- Bone-metastatic context and absence of known visceral disease (per indication).
- Marrow reserve and concurrent therapies that increase cytopenia risk.
- Define goals of therapy and monitoring plan for symptomatic skeletal events.
These are workflow reminders, not prescribing rules.
NuDat (NNDC, Brookhaven)
NuDat is a web application that allows users to search and plot nuclear structure and nuclear decay data interactively. It was developed by the National Nuclear Data Center (NNDC) at Brookhaven National Laboratory.
Open NuDatReferences
- Sartor O, et al. Lu-PSMA-617 for metastatic castration-resistant prostate cancer (VISION). N Engl J Med. 2021.
- Hofman MS, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in mCRPC (TheraP). Lancet. 2021.
- Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer (ALSYMPCA). N Engl J Med. 2013.
- NNDC (Brookhaven National Laboratory). NuDat 3.0 — interactive nuclear structure and decay data. nndc.bnl.gov/nudat3.
Agent-specific references will be added as the page evolves.